
Carbon dioxide, water vapor, and several other important greenhouse gases contribute to global warming via the so-called greenhouse effect.
There's a whole family of greenhouse gases (GHGs). But an important thing to remember is that they are not all created equally.
An important distinction among them is their varying Global Warming Potentials (GWPs). Some are much more “efficient”—decidedly not a compliment in this context—at warming the planet by preventing infrared radiation from escaping to space. Some are short-lived, while others can easily last decades or longer in the atmosphere.
Some GHGs are emitted in vast quantities but, fortunately, may not be so voracious or efficient as those emitted in far smaller quantities. Others have exactly the opposite qualities—emitted in only trace amounts but extremely efficient in blanketing the planet's atmosphere and keeping heat from escaping beyond it.
To bring some understandable reason to the family of GHGs, scientists speak in terms of carbon dioxide equivalent (CO2e). This approach makes carbon dioxide, CO2, the prevailing “currency” of greenhouse gases and global warming.
Let's consider the principal GHGs one at a time, starting with water vapor, the most abundant greenhouse gas in the atmosphere according to NOAA's Climate.gov. To skip to a specific section, click on its link below.
- Water Vapor
- Carbon Dioxide (CO2)
- Methane (CH4)
- Nitrous oxide (N2O)
- Fluorinated Gases (HFCs, PFCs, SF6,NF3)

Water Vapor
Water vapor is the most abundant of the greenhouse gases. While it is not directly produced by industrial activity, a warming atmosphere will become more humid. The net effect of an increase in water vapor on our climate is nuanced.
On the one hand, because water vapor is a greenhouse gas, more water vapor could amplify the greenhouse effect and lead to further warming. On the other hand, increasing the humidity may result in more widespread cloudiness, thus reflecting more sunlight and cooling the Earth’s surface. Understanding and quantifying these competing effects is critically important to projecting future climate change.
Significant progress has been made in recent years towards quantifying the net effect of clouds and water vapor on global warming. Better observations and advancements in computer model simulations have helped reduce the uncertainty. According to the Intergovernmental Panel on Climate Change (IPCC) 6th Assessment Report, the net effect is to amplify the warming caused by human activities.

Carbon dioxide (CO2)
Let's now consider what federal agency and academic researchers consider to be the “most important greenhouse gases.”
Carbon dioxide (not to be confused with carbon monoxide, CO, associated with vehicle tailpipe emissions or with home CO alerts) occurs both naturally and as a result of human activities.
CO2 is produced, consumed, and stored via natural processes involving decaying organic matter, plants and animals, the land, the ocean, and the atmosphere. These natural sources and sinks are typically in balance. Since the start of the industrial age, CO2 concentrations in the atmosphere have risen dramatically, and they continue to do so, mainly due to combustion of fossil fuels, such as coal, gasoline, and natural gas.
In 2021, CO2 accounted for about 79% of all US greenhouse gas emissions from human activities. The IPCC 6th Assessment Report states that “In 2019, atmospheric CO2 concentrations (410 parts per million) were higher than at any time in at least 2 million years.”
The continuing upward trajectory of CO2 concentrations under a “business as usual” scenario is one of the matters of particular concern to climate scientists.
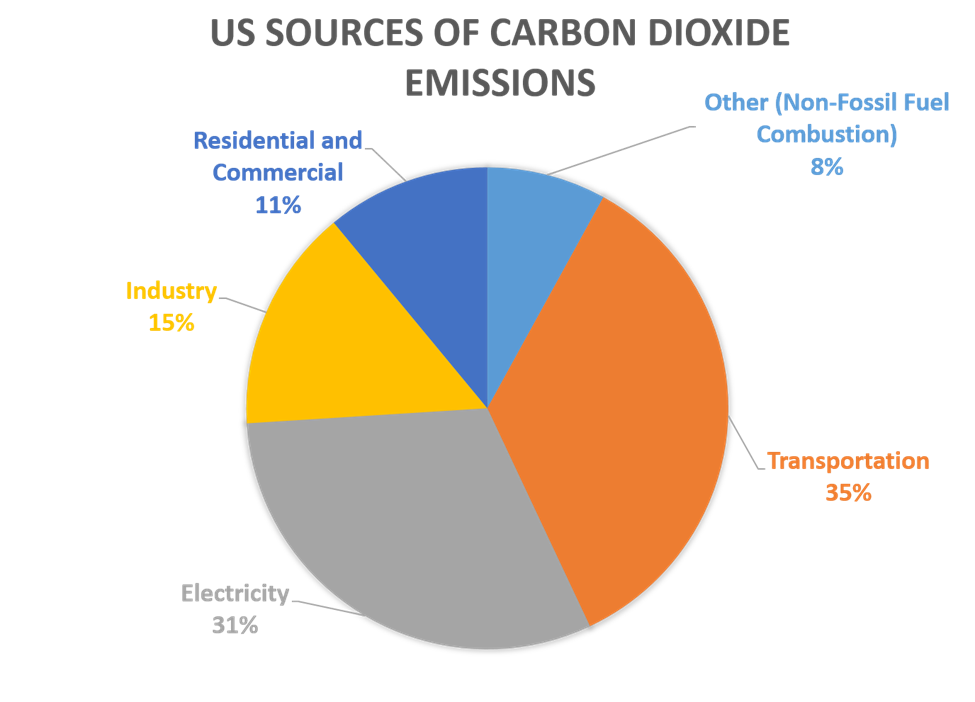
Source: EPA, Inventory of US Greenhouse Gas Emission and Sinks : 1990-2022
It's not so much the GWP of carbon dioxide that is concerning—it’s the current and projected growth in emissions and atmospheric concentrations, and the fact that CO2 is very long-lived (in some cases more than a century) in the atmosphere. What we emit today is going to remain in the atmosphere for a very, very long time.
Carbon dioxide is, of course, critical to plant growth and food production, and it's emitted each time we humans exhale. In the atmosphere, however, it's a case of too much of a good thing: The science community has known since the research findings of Swedish scientist and Nobel Laureate Svante Arrhenius more than a century ago that humans' burning of fossil fuels leads to a greenhouse effect caused by the release of CO2.
For more information, see Understanding the Emphasis on CO2 as a Greenhouse Gas.

Methane (CH4)
Methane, a hydrocarbon gas resulting from both natural causes and as a result of human activities such as agriculture and farming, is an especially potent GHG and absorber of radiation. Valued for energy production, methane, like CO2, is odorless and colorless—and it has both beneficial and harmful qualities.
Methane is far less abundant than CO2 in the atmosphere, and it has a considerably shorter lifespan of 12 years. Nevertheless, as of 2016, methane accounted for approximately 20% of the total GHG-related radiative forcing. As of 2021, methane concentrations had risen to levels that were 240% of their pre-industrial values.
EPA figures indicate that human activities account for 50-65% of total methane emissions globally, primarily from industry, agriculture, and waste management activities. This chart shows methane contributions by various sources in the US:
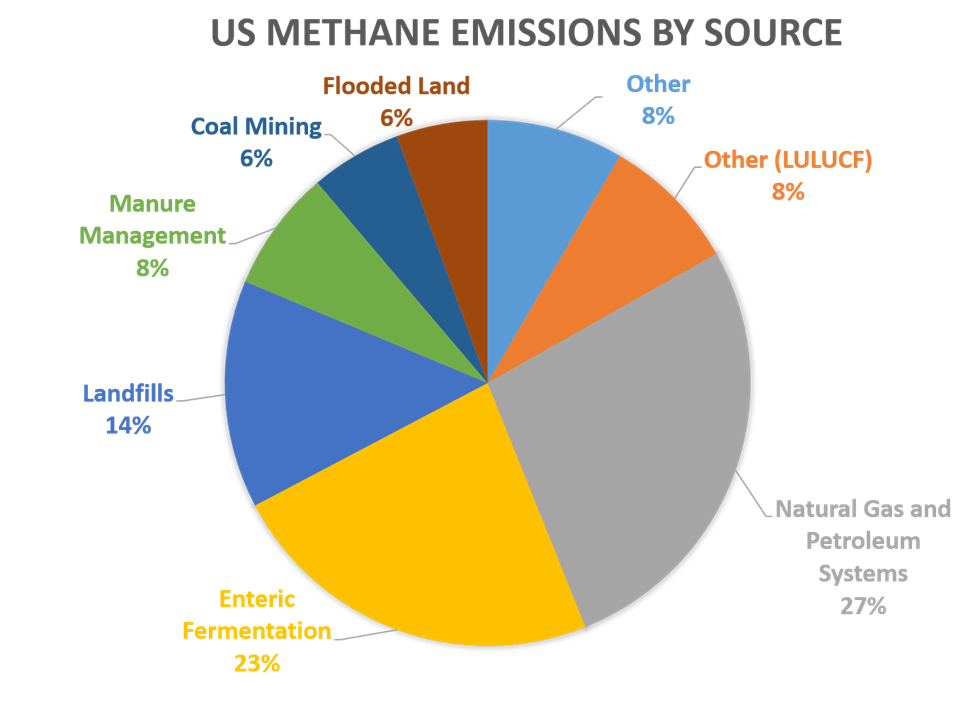
Source: EPA, Inventory of US Greenhouse Gas Emission and Sinks : 1990-2022
According to the US Environmental Protection Agency (EPA), wetlands are the largest natural source of methane, emitting it from bacteria that decompose organic materials in the absence of oxygen. Smaller sources include termites, oceans, sediments, volcanoes, and wildfires.
NOAA’s Global Monitoring Laboratory reports that methane concentrations have continued to rise after a short-lived pause between 2000 and 2007. The majority of this increase (about 60%) has been due to human activities.
Over the past few decades, some media reports have focused increased attention on the potential for sudden and massive releases of long-bottled-up methane and methane hydrates currently sequestered by frozen tundra. The concern is that melting of the Arctic tundra could lead to potentially catastrophic and abrupt releases of methane.
An excellent resource for further understanding this high-visibility issue is a report published in the widely respected peer-reviewed journal Nature by US Geological Survey (USGS) and Woods Hole Coastal and Marine Science Center researcher Dr. Carolyn Ruppel. Here is a quote from that report:
Some scientists raised the alarm that large quantities of methane (CH4) might be liberated by widespread destabilization of climate-sensitive gas hydrate deposits trapped in marine and permafrost-associated sediments (Bohannon 2008, Krey et al. 2009, Mascarelli 2009). Even if only a fraction of the liberated CH4 were to reach the atmosphere, the potency of CH4 as a greenhouse gas (GHG) and the persistence of its oxidative product (CO2) heightened concerns that gas hydrate dissociation could represent a slow tipping point (Archer et al. 2009) for Earth's contemporary period of climate change.
Noting that methane is about 20% more potent greenhouse gas than CO2 but oxidizes to CO2 after about a decade in the atmosphere, Ruppel writes that “The susceptibility of gas hydrates to warming climate depends on the duration of the warming event, their depth beneath the seafloor or tundra surface, and the amount of warming required to heat sediments to the point of dissociating gas hydrates.”
For those wanting to better understand the significance of methane in the global warming/climate change discussion, the full Nature piece by Dr. Ruppel, chief of USGS's Gas Hydrates Project, provides useful and practical information.

Nitrous oxide (N2O)
Nitrous oxide occurs naturally in Earth's atmosphere as part of the nitrogen cycle. While it is the product of a wide variety of natural sources, human activities such as agriculture, fossil fuel combustion, wastewater management, and industrial processes are increasing atmospheric concentrations according to the EPA.
In addition, nitrous oxide molecules in the atmosphere have long life spans (about 120 years) before they are removed or destroyed as a result of chemical reactions. A pound of N2O gas has the equivalent warming effect of 300 times that of one pound of carbon dioxide.
Based on 2021 data, nitrous dioxide comprises about 6% of all US emissions resulting from human activities. Globally, about 40% of nitrous oxide emissions are attributable to human activities.
Agriculture, transportation, and industry activities are major sources of nitrous oxide emissions, as indicated on this chart:
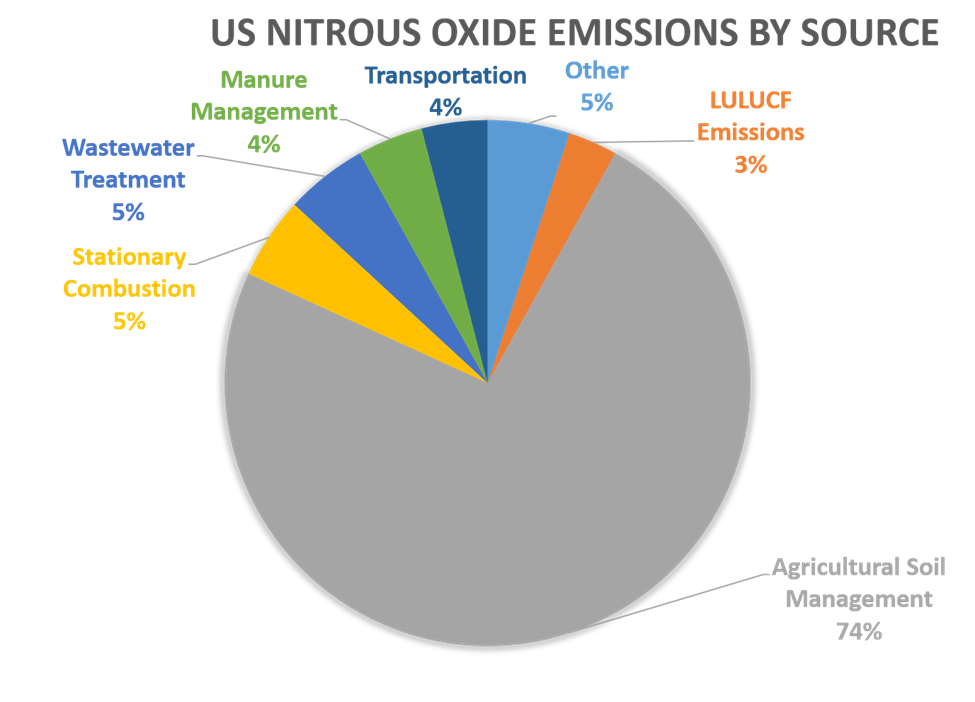
Source: EPA, Inventory of US Greenhouse Gas Emission and Sinks : 1990-2022
Fluorinated Gases (HFCs, PFCs, SF6, NF3)
There are four main types of fluorinated gases—hydrofluorocarbons (HFCs), perfluorocarbons (PFCs), sulfur hexafluoride (SF6), and nitrogen trifluoride (NF3).
Fluorinated gases are emitted in smaller quantities than the other greenhouse gases, but what they lack in volume they can make up in potency and long lifespans. This ranges from 1-270 years for HFCs to 800-50,000 years for PFCs to about 3,200 years for SF6.
Once emitted into the atmosphere, these gases disperse widely around the globe—they are removed from the atmosphere only by sunlight in the highest levels of the atmosphere. Being the most potent of the GHGs and having the longest lifespans, these gases are often described as “high global warming potential (GWP) gases.”
Aluminum and semiconductor manufacturing processes are among the principal emitters of the fluorinated gases, as illustrated by this chart:
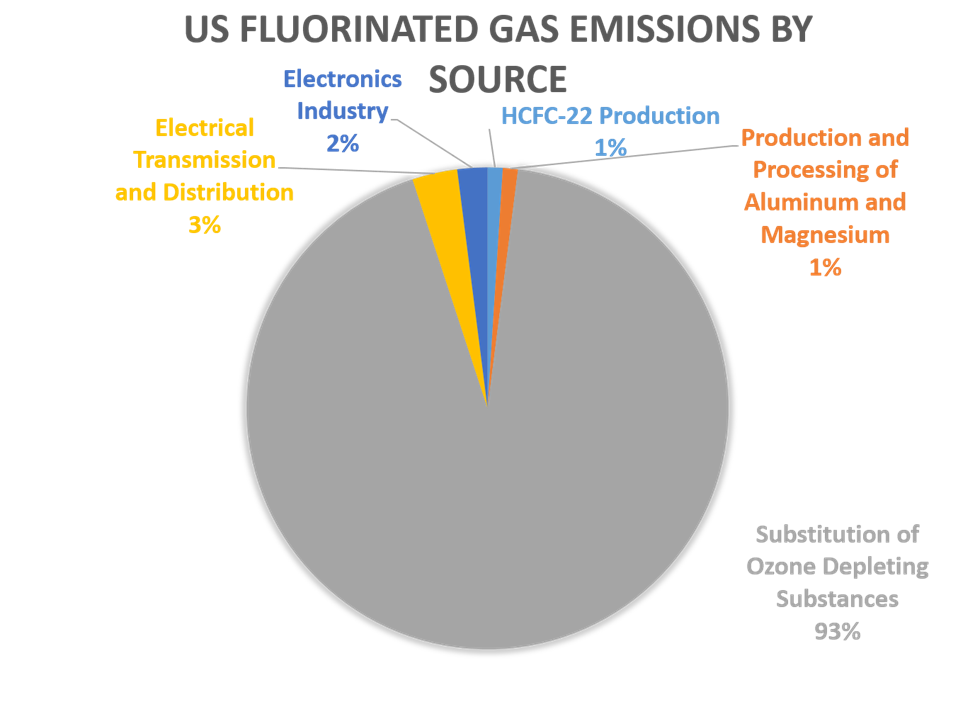
Source: EPA, Inventory of US Greenhouse Gas Emission and Sinks : 1990-2022
Future Climate Change and Greenhouse Gases
Greenhouse gases have been increasing in concentration since the beginning of the Industrial Age. The enhanced greenhouse effect due to these gases has contributed significantly to global warming. If the world were to decide tomorrow to drastically limit the emission of greenhouse gases, would this warming instantly pause? Unfortunately, the answer is no.
Fortunately, there are steps we all can take to understand climate change and its effect on things like animal migration, infrastructure, and human health. The more we know, the better equipped we are to address the impacts of climate change. You can learn more by exploring the Climate Change section of NEEF’s website.
About the Authors
Morris A. "Bud" Ward, former editor of Yale Climate Connections, is a proven and widely experienced communicator and educator on environmental, energy and climate change issues. He has an extensive publishing history including hundreds of bylined news and analysis articles and authorship or co-authorship of five professional books. He has conducted numerous first-hand workshops for reporters, editors and policy makers on issues involving journalism/communications, climate change and environmental risk. An elected fellow of the American Association for the Advancement of Science (AAAS), he is a member of SEJ and of the American Geophysical Union (AGU) and the American Meteorological Society (AMS). He earned bachelor’s and master’s degrees from Penn State University.
Jeffrey Chagnon is a member of faculty in the Earth, Ocean, and Atmospheric Science Department at Florida State University. He received his Ph.D. in Meteorology from Penn State University and has published over twenty peer-reviewed research articles in high impact journals. He is a passionate educator whose dedication to his students earned him a University Teaching Award from Florida State University in 2022.


